RapidXAFS Results: Benchtop XAFS shines in the field of electrocatalysis
X-ray Absorption Fine Structure (XAFS) is an important method to study the local structure of materialsXAFS testing can be completed without synchrotron radiation light source, meeting the daily sample analysis in the laboratory, and in-situ detection of the reaction process under various atmospheric conditions such as high temperature and high pressure. XAFS is very sensitive to the local structure and chemical environment of the central absorbing atom, so it can give the structural information of several adjacent coordination shells around a characteristic atom at the atomic scale, such as the type of coordination atom, coordination number and atomic spacing and other structural parameters, which is widely used in catalysis, energy, nanomaterials and other popular fields.
However, for a long time, XAFS can only be tested on various synchrotron radiation light sources, which is difficult to meet the growing testing needs of scientific researchers. Adhering to the concept of bringing XAFS technology into every laboratory, Anhui Absorption Spectrometer Equipment Co., Ltd. has launched a new desktop X-ray absorption fine structure spectrometer RapidXAFS. With its extremely high sensitivity and light source quality, RapidXAFS can obtain XAFS data comparable to that of synchrotron radiation source XAFS, and now the valence analysis and coordination structure analysis of elements are now carried out.
Fig.1 Physical diagram of benchtop X-ray absorption fine structure spectrometer
RapidXAFS Advantages:
High performance: Delivers research-grade, high-quality XAFS data
Energy range: 4.5-15 keV, expandable to 20 keV
High luminous flux: up to 2,000,000 photons/sec@8 keV
Low Concentration Sample Testing: The C-N vector can be tested with 0.1 wt% sample XANES and 0.5 wt% sample EXAFS tested
Benchtop design: XAFS testing can be completed without synchrotron radiation light source, which can meet the daily sample analysis in the laboratory
In-situ test: (high temperature and high pressure, low temperature and low pressure, under various atmospheric conditions), in-situ detection of the reaction process
Low maintenance cost: no need for special personnel to maintain, operate, manage, etc
Representative Articles
Since the establishment of the company in May 2022, RapidXAFS has sold 20 sets of equipment in domestic units, and has been published in the fields of battery, catalysis, and environment44A high-level SCI article. Among them, J. Am. Chem. Soc., Nat. Commun.,Adv. Funct. Mater.,ACS Catal.,Angew. Chem. Int. Edit.,Appl. Catal. B-Environ.More than 80% of high-level SCI papers and more than 70% of articles with sample content below 5%. Recent representative articles published by researchers in the field of electrocatalysis using RapidXAFS include:
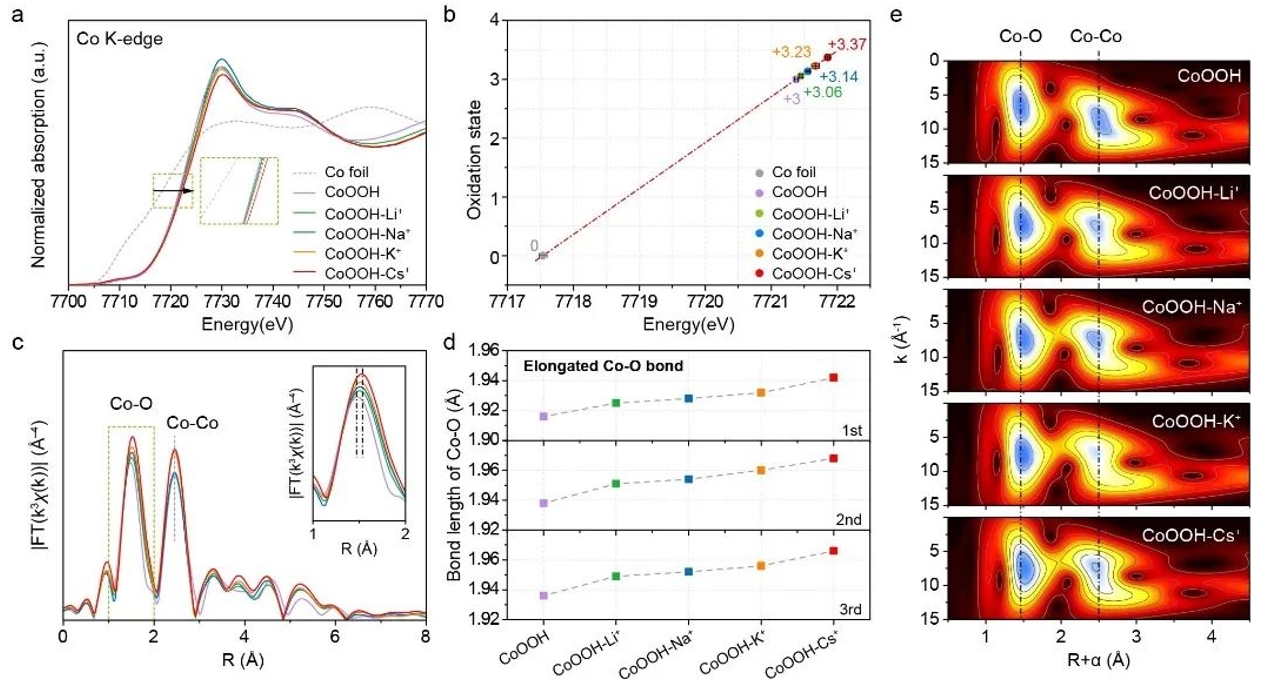
Chapter 1 First In-situ OER Work: Cation-Dependent In-situ Kinetics of OER Electrolytes (Angew Chem Int Ed. e202216835)
The development of efficient and renewable energy conversion devices is expected to alleviate the energy crisis caused by the excessive consumption of fossil fuels, and electrocatalysis can realize the mutual conversion of chemical energy and electrical energy, so it is considered to be a promising sustainable energy storage and utilization solution. At present, the correlation between catalytic activity and electrolyte cation has been widely observed in many fields of electrocatalysis, but the exact mechanism of its influence on catalytic activity is still unclear.
Recently, Professor Luo Wei's team from Wuhan University held a conference in Angew. Chem. Int. Edit., "Unveiling the Electrolyte Cations Dependent Kinetics on CoOOH-Catalyzed Oxygen Evolution Reaction", which focuses on the microenvironmental changes of catalytic sites caused by alkali metal cation intercalationThe local atomic and electronic structure of CoOOH-M+ was characterized by the benchtop XAFS (RapidXAFS 2M) of Anhui Absorption Spectroscopy Instrument Equipment Co., Ltd., which confirmed the increase in the valence state of CoOOH-M+ caused by cation insertion, and further confirmed the law that the average bond length of Co-O bond lengthens with the increase of electrolyte cation radius through fitting, and then has better OER reaction kinetics, and establishes " dynamic structure-adsorption capacity-catalytic activity".
Fig.2 In-situ XAFS analysis and structural study of CoOOH-M+
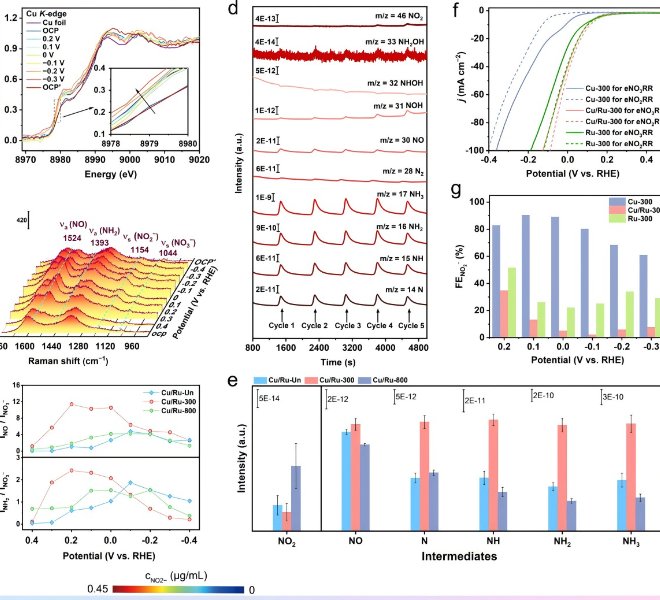
Chapter 2 First In-situ CO₂RR Work: Pulsed Electrolysis Promotes CO₂ Reduction of Cu₂O/Ag Heterostructure Catalyst CO₂ to Ethanol (Small, 2307637)
The electrochemical conversion of CO₂ into specific high-value products is essential for achieving a carbon-neutral economy. Compared to C₁ products (CO, HCOOH,CH 4, etc.), ethanol (EtOH) is attracting attention as a representative multi-carbon (C2+) product with high energy density. To date, copper and copper-based catalysts have been able to produceC2+ products, and during the CO₂ reduction reaction (CO₂RR), copper-based catalysts produce two to three times more ethylene than ethanol. Adjusting the ethanol-to-ethylene ratio remains challenging due to the largely overlapping reaction pathways of ethanol and ethylene, which requires fine-tuning of the catalytic site and local environment.
Recently, the team of Professor Wu Haobin of Zhejiang University published a paper entitled "Pulsed Electrolysis Promotes CO₂ Reduction to Ethanol on Heterostructured Cu₂O/Ag Catalysts" in SmallIn this article, we report a highly efficient catalyst with a rich Cu₂O/Ag interface for the preparation of ethanol by pulsed CO₂ electrolysis, consisting of Cu₂O hollow nanospheres (se-Cu₂O/Ag) supported with Ag nanoparticles. In the neutral flow cell, the Faraday efficiency of the ethanol electrolyzed by pulsed CO₂ electrolysis is significantly increased to 46.3%, and the current density is as high as 417 mA cm-2The performance is significantly better than that of traditional static electrolytic Cu catalysts. In order to further explore the mechanism, the authors used the desktop XAFS (RapidXAFS 2M) of Anhui Absorption Spectroscopy Instrument Equipment Co., Ltd. to analyze the reaction process, and the results showed that the Cu+ component of Se-Cu₂O/Ag was stable during the pulse electrolysis process, which improved the coverage of the adsorbed CO intermediate (*CO), and the Cu was stable under the pulse electrolysis condition+ Hydrogenation of HCCOH intermediates (*HCCOH) that is conducive to adsorption to adsorbed HCCHOH intermediates (*HCCHOH) is beneficial for the generation of ethanol.
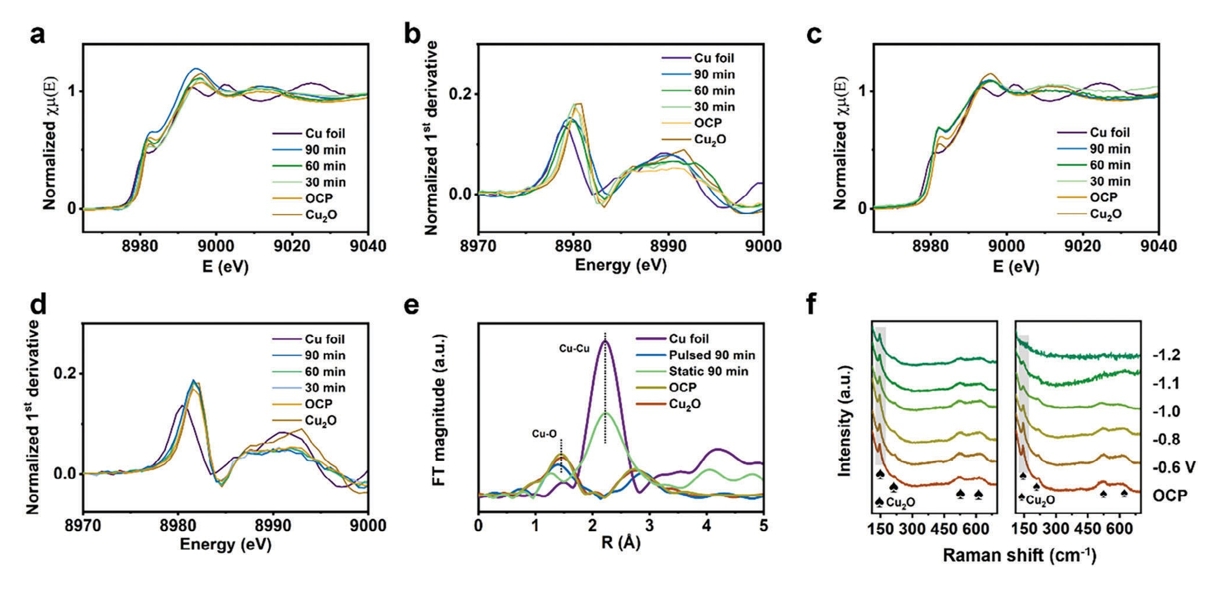
Fig.3 In-situ XAFS analysis of Se-Cu₂O/Ag
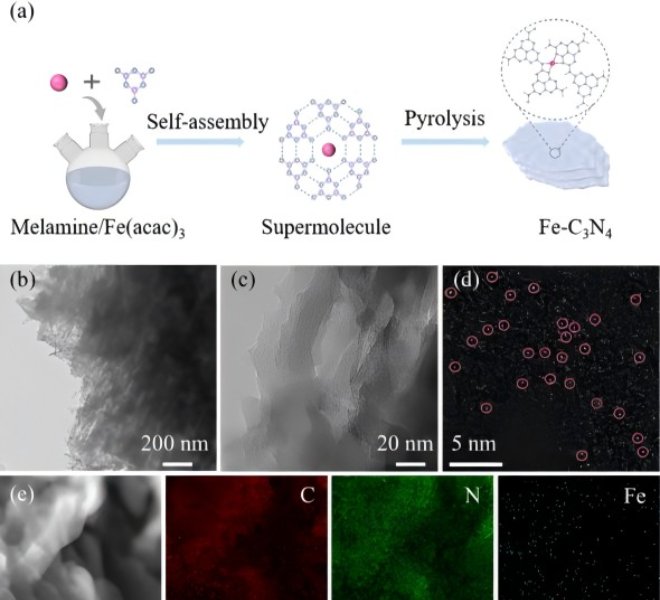
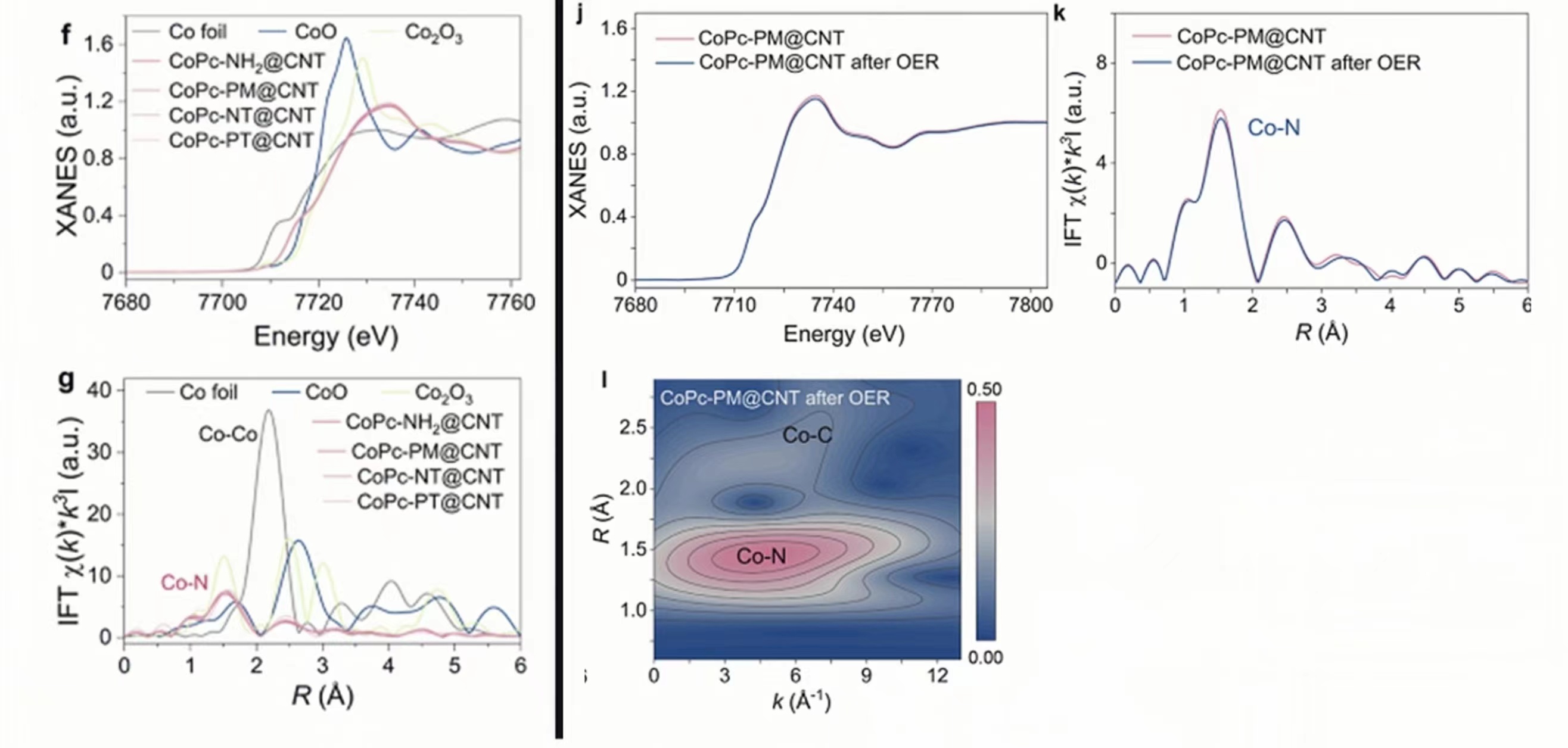
Chapter 3 Molecular Interval Optimization of Spatial Density of Single Atomic Co Sites for Sustainable Electrocatalytic Water Oxidation (J Am Chem Soc, 3c06665)
The study of single-atom catalysts (SACs) provides a new solution for the design and synthesis of high-efficiency electrocatalysts with high activity and plateau utilization. One of the common strategies used to improve SAC performance is to increase the loading of metal atoms. However, as the metal loading increases, the agglomeration of metals or active centers is unavoidable, which will limit the further increase in the effective active density (or actual atom utilization) of the catalyst.
Recently, the team of Professor He Chunting of Jiangxi Normal University published a paper entitled "Optimizing Spatial Density of Single Co Sites via Molecular Spacing for Facilitating Sustainable Water Oxidation" in J Am Chem SocIn this paper, a series of different organic anhydrides are used as spacer molecules to regulate the spatial density of a single Co site in discrete metal phthalocyanine, and novel molecular-based single-atom catalysts (msSACs) are designed and synthesized, which significantly improve the effective active site density and mass transfer performance, thereby changing the apparent activation energy of the catalysts. In this work, the authors used a benchtop XAFS (RapidXAFS 1M) from Anhui Absorption Spectroscopy Instrument and Equipment Co., LtdThe characterization of three different organic anhydrides as spacer molecules did not significantly change the electronic structure and coordination environment of Co, confirming that the spacer density of single-atom sites was mainly optimized by the molecular spacer strategy. On the other hand, XAFS spectroscopy also confirmed that the coordination environment of CoPc-PM@CNT remained unchanged before and after the OER reaction, which corroborated the excellent long-term stability test results of such msSACs.
Fig.4 Left: XAFS spectra of Co in three spaced monoatomic catalysts, right: XAFS spectra and wavelet transform of Co before and after CoPc-PM@CNT OER reaction
Chapter 4 Dual-core copper(I) sites confined to a monometal-organic layer promote the electroreduction of CO2 to CH4 in neutral electrolytes (J Am Chem Soc, 3c08571)
The overuse of traditional fossil fuels such as coal, oil and natural gas has led to excessive carbon dioxide emissions, leading to a series of environmental problems that need to be solved urgently. Electrochemical CO₂ reduction reactions (eCO₂RR) powered by renewable electricity provide an effective strategy for utilizing CO₂ and reducing CO₂ emissions. Normally eCO₂RR toCH4 is carried out in a strong alkaline electrolyte, but under alkaline conditions, CO₂ will react with OH– to form carbonates, resulting in significant CO₂ loss and increasing the likelihood of gas diffusion channel blockage. Therefore, the development of catalysts that can achieve high-performance conversion under neutral or acidic conditions will have great application potential.
Recently, Professor Liao Peiqin's team from Sun Yat-sen University published a paper entitled "Dicopper(I) Sites Confined in a Single Metal–Organic Layer Boosting the Electroreduction of CO₂ to CH4 in a Neutral Electrolyte" in J Am Chem Soc In this paper, we analyzed the efficient production ofCH4 in a neutral aqueous solution by the active site of monolayer metal-organic layer nanosheets under the action of the active site of dual-core copper(I) and showed no significant reduction in performance after 100 hours of continuous operation. The authors used the benchtop XAFS (RapidXAFS 1M) of Anhui Absorption Spectroscopy Instrument Equipment Co., Ltd. to characterize that no significant changes were observed in Cuobpy-SL before and after electrolysis, demonstrating the stability of Cuobpy-SL.
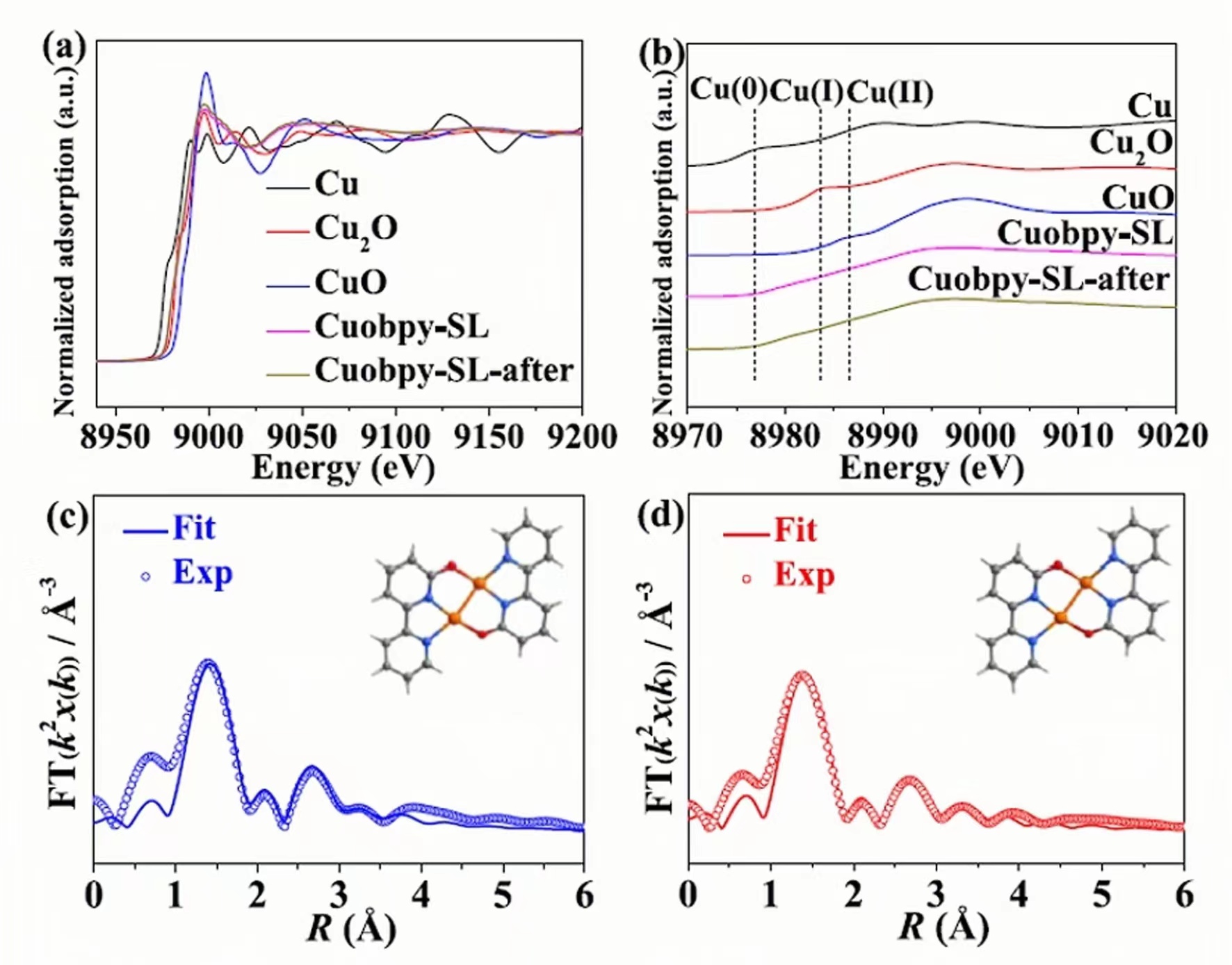
Fig.5 XAFS spectra of Cu before and after Cuobpy-SL electrolysis
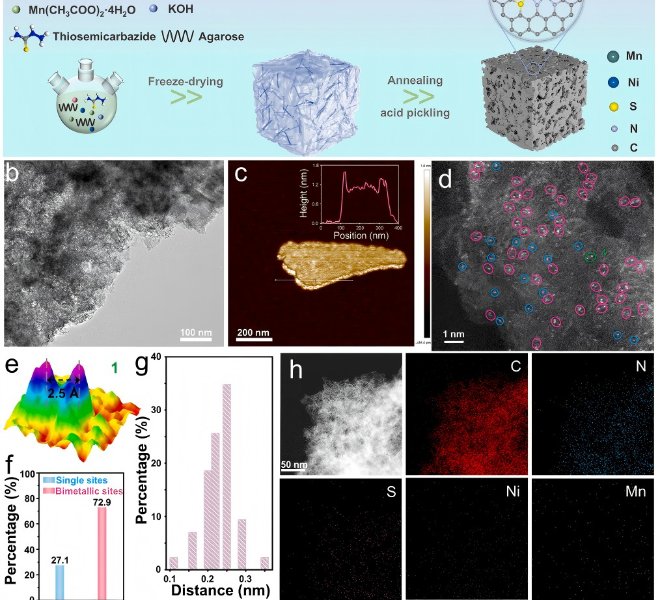
Chapter 5 Revealing the Role of Hydrogen Peroxide in pH-Dependent ORR Performance of Mn-N-C Catalysts (Appl Catal B: Environ, 123458)
Under the operating conditions of proton exchange membrane fuel cell (PEMFC), the Fe-N-C transition metal catalyst is considered to be the most promising platinum group metal (PGM)-free ORR catalyst, but its electrochemical stability is poor, and Mn-N-C, as a catalyst with lower Fenton reactivity, has made significant progress in synthesis technology and performance improvement. So far, the Mn-N-C catalyst has shown high activity in alkaline media, but its performance in acidic medium is poor. Understanding its interesting but confusing strong pH-dependent activity facilitates the rational design of high-performance Mn-N-C catalysts. Recently, Professor Jiannan Zhang's team from Zhengzhou University published a paper entitled "Unravelling the role of hydrogen peroxide in pH-dependent ORR performance of Mn-N-C catalysts" at Appl Catal B: EnvironIn this work, the authors synthesized Mn-N-C catalysts by loading manganese phthalocyanine molecules (MnPc) onto porous carbon black and then heat treatment. The coordination structure of the active center was characterized by the benchtop XAFS (RapidXAFS 2M) of Anhui Absorption Spectroscopy Instrument Equipment Co., Ltd., and the results of electron microscopy proved that Mn was atomically dispersed in the MnPc/C-300 catalyst, embedded in the support and coordinated with four N atoms in the neighbors.
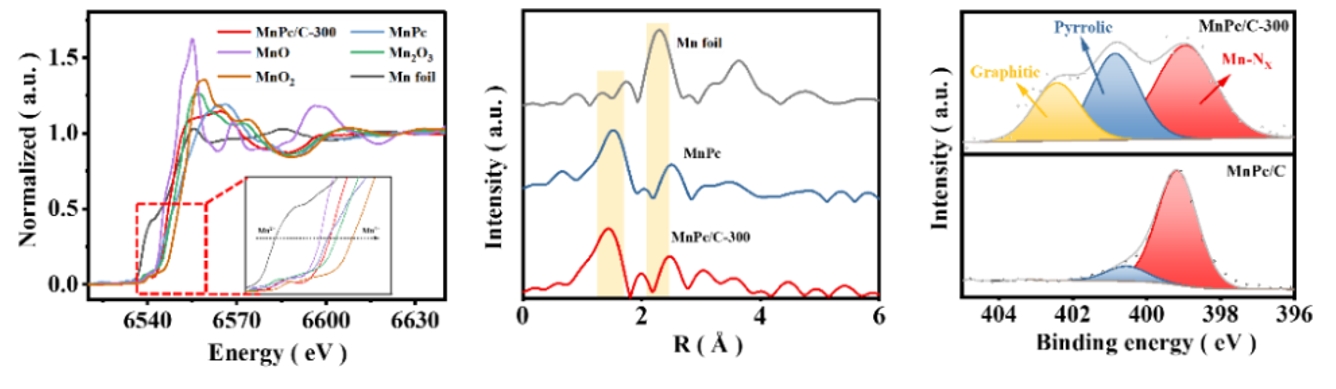
Fig.6 XAFS spectra of MnPc/C-300
Some of the articles have been published
1. Liao P., et al (2024).. J Am Chem Soc, 3c12423.
2. Guo, H., et al (2023).. Adv Mater, 202304511.
3. Zhu. J., et. al (2023).. Adv Mater, 202209644.
4. Chen, X. et al (2023).. J Am Chem Soc, 3c08571.
5. He, C. et al (2023). J Am Chem Soc, 3c06665.
6. Zheng, L. et al (2023). J Am Chem Soc, 3c05552.
7. Luo, W. et, al. (2023). Angew Chem Int Ed. e202216835.
8. Yang, J. et, al. (2023). Angew Chem Int Ed. e202315834.
9. Wang, X. et, al. (2023). Angew Chem Int Ed. 202313886.
10. Li, L., et al (2023). Angew Chem Int Ed, e202307160.
11. Xu Y., et al. (2023). Angew Chem Int Ed, e202317664.
12. Wu H., et, al. (2023). Small, 2307637. (First article on in-situ CO₂RR reaction)
......